

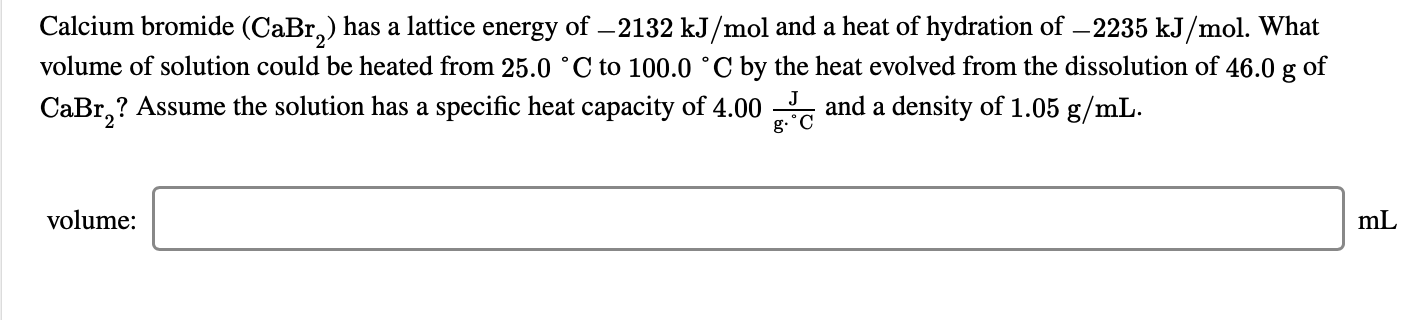
#CuSO(aq)+BaCl_2(aq)toCuCl_2(aq)+BaSO_4(s) DeltaH =-17.7 kJmol^-1#Ĭalculate the temperature change when #900cm^3# of 1M copper (II) sulphate were added to 600#cm^3# of 1M barium(II)chloride.(Assume Copper (II) sulphate reacts with barium chloride according to the equation below. Use the following equations to determine the heat evolved when Aluminium metal is reacted with iron(III)oxide. (c ) When pure water is heated at 1 atmospheric pressure at sea level, the temperature of the water does not rise beyond #100^oC#,even with continued heating. (b) Changes of state are either exothermic or endothermic. (a) Distinguish between exothermic and endothermic reaction. (b) Calculate the heat change for the process: Use the equations below to answer the questions that follow. (Specific heat capacity of solution=#4.2Jg^-1K^-1# density of water=#1gcm^-3# O=16 H=1) Determine the rise in temperature due to the #H_2O_2(l)toH_2O(l)+1/2 O_2(g) DeltaH =-98kJ#Ĩ.5 g of hydrogen peroxide contained in #100cm^3# of solution with water were completely decomposed. Hydrogen peroxide decomposes according to the equation below In the space provided, sketch a simple energy level diagram for the above change. At 298K and 1 atmosphere, graphite changes into diamond according to the equation:Ĭ(graphite)?C(diamond) #DeltaH=2.9kJmol^-1# (b) Calculate the molar enthalpy of formation of HF. (a) On the grid provided below, sketch the energy level diagram for the forward reaction. Hydrogen and Flourine react according to the equation below: Use the information below to answer the question that follows.Ĭalculate the enthalpy change for the reaction CaO(s)+#CO_2#(g)#to#CaCO_3#(s) The temperature of the resulting solution was recorded afterĮvery 30 seconds until the highest temperature of the solution was attained. In order to determine the molar heat of neutralization of sodium hydroxide, 100#cm^3# of 1M sodium hydroxide and 100#cm^3# of 1M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. study the information in the table below and answer the questions below the tableĬalculate the enthalpy change of the reaction: Calculate the relative atomic mass of element J given that the specific heat capacity of water=#4.2KJ^(-1)g^-1#, density of water=#1.0gcm^-3# and molar When 0.6g of element J were completely burnt in oxygen and all the heat evolved was used to heat #500cm^3# of water, the temperature of the water rose from #23^0C# to #32^0C#. (b) Write an expression, for #DeltaH_3# in terms of #DeltaH_1# and #DeltaH_2# Study the diagram and answer the questions that follow. A simple energy level diagram for the reaction is given below. Sulphur burns in air to form sulphur (IV) oxide.

(b) Calculate the molar enthalpy of combustion, #DeltaH4# of ethanol (a) Define the term “enthalpy of formation” of a compound. Use the information below to answer the questions that follow: In an experiment determine the heat of combustion of methanol, #CH_3OH#, a student used a set up like the one shown in the diagram below.


 0 kommentar(er)
0 kommentar(er)
