
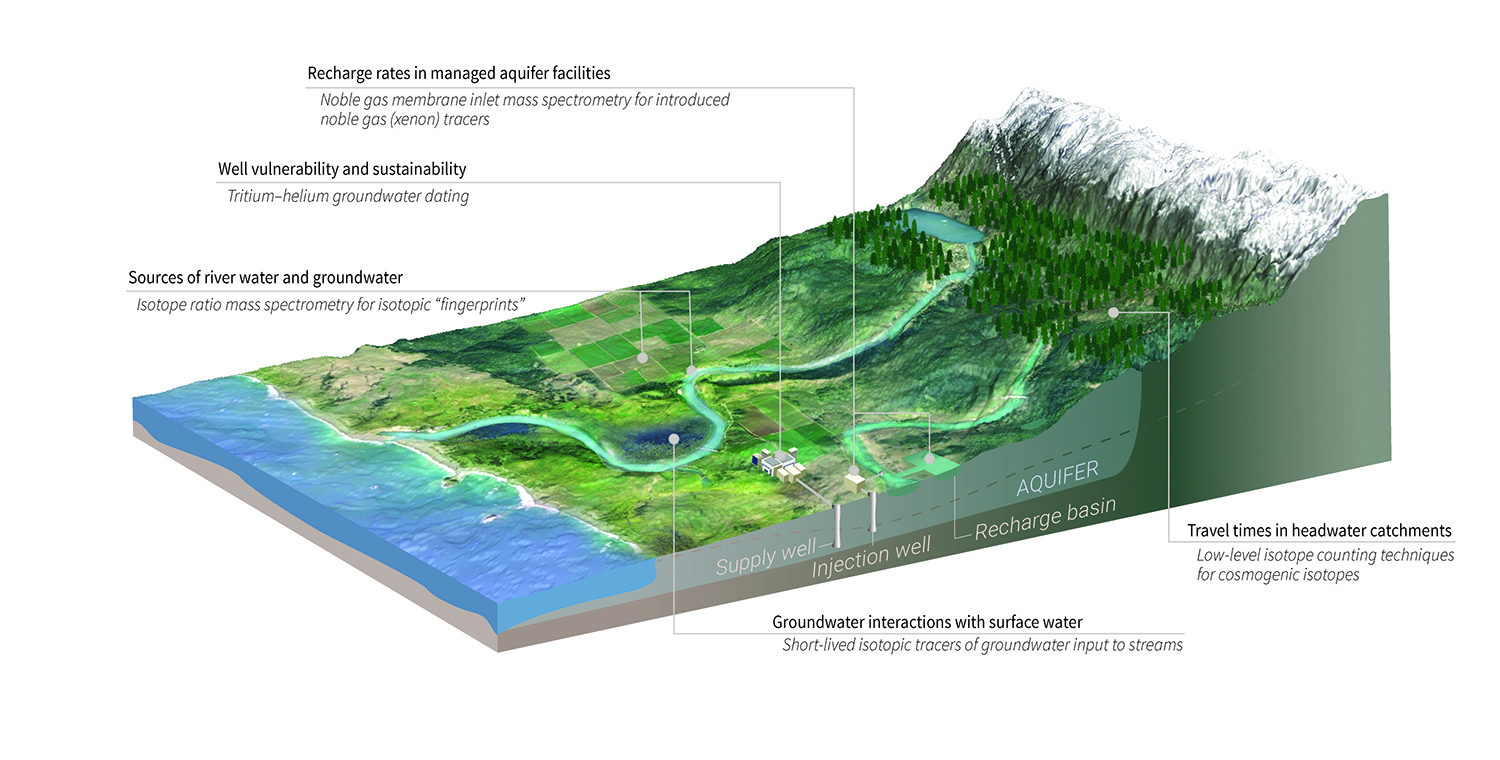

Write isotopic symbols in the form for each. The atomic number is sometimes written as a subscript preceding the symbol, but since this number defines the element’s identity, as does its symbol, it is often omitted. Isotopes are forms of the same element with equal numbers of protons but. The symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol (Figure 4).
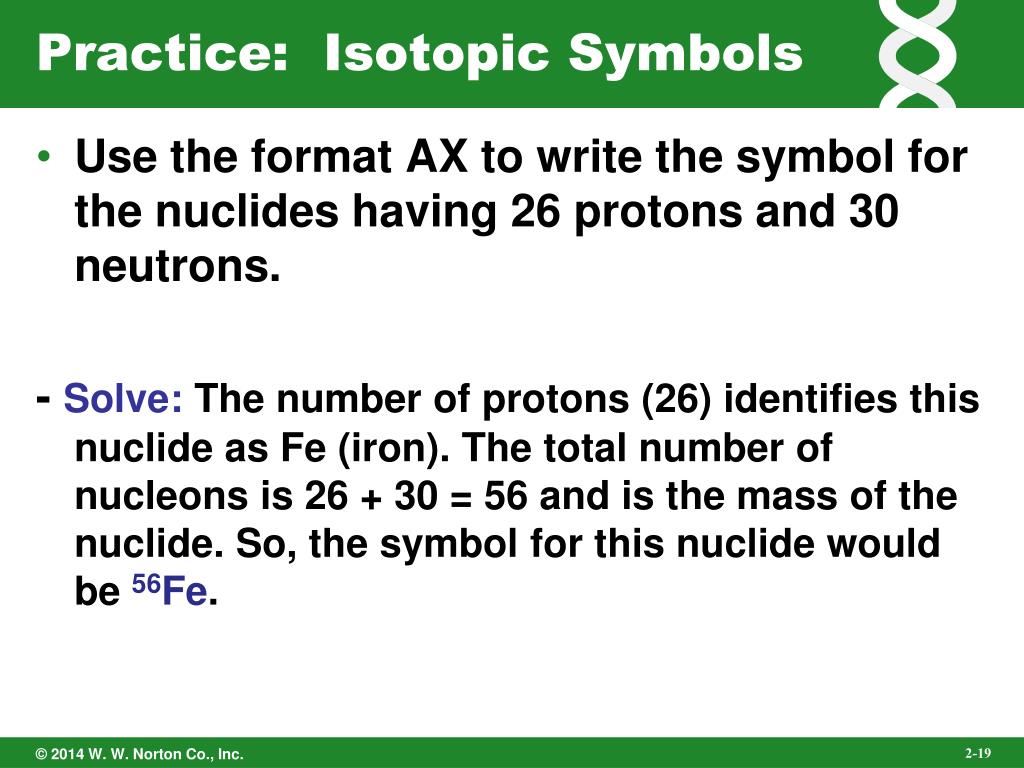
Introductory Chemistry (6th Edition) 6th Edition. How do I get the chemical symbol Si in roman, and the superscript and symbol. Instead, you have to figure it out based on the number of protons or atomic number. It does not indicate the number of electrons. When an organism dies, it stops taking in carbon-14, so the ratio of carbon-14 to carbon-12 in its remains, such as fossilized bones, will decline as carbon-14 decays gradually to nitrogen-14 2 ^2 2 squared. Which pair of elements do you expect to be most similar Why a. The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element.
Si element isotopic symbol how to#
As animals eat the plants, or eat other animals that ate plants, the concentrations of carbon-14 in their bodies will also match the atmospheric concentration. 0:00 / 4:12 The Periodic Table How to write in Isotopic Symbol - Dr K ChemSimplified 12K subscribers 13K views 3 years ago In this video, we are going to go through how to write in isotopic. As plants pull carbon dioxide from the air to make sugars, the relative amount of carbon-14 in their tissues will be equal to the concentration of carbon-14 in the atmosphere. These forms of carbon are found in the atmosphere in relatively constant proportions, with carbon-12 as the major form at about 99%, carbon-13 as a minor form at about 1%, and carbon-14 present only in tiny amounts 1 ^1 1 start superscript, 1, end superscript. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and. Where the element is most commonly found in nature, and how it is sourced commercially. The role of the element in humans, animals and plants. The description of the element in its natural form. For example, carbon is normally present in the atmosphere in the form of gases like carbon dioxide, and it exists in three isotopic forms: carbon-12 and carbon-13, which are stable, and carbon-14, which is radioactive. Element Silicon (Si), Group 14, Atomic Number 14, p-block, Mass 28.085. Isotopes are different atoms of the same element that contain the same number of protons but a different number of neutrons The symbol for an isotope is the. This is where the artist explains his interpretation of the element and the science behind the picture.


 0 kommentar(er)
0 kommentar(er)
